Theoretical and Experimental Biocatalysis
Tematyka badawcza
MAIN RESEARCH TOPICS
-
Investigation of substrate spectrum of enzymes; analysis of reaction products.
-
Studies of kinetics and thermodynamics of enzyme’s reduction and oxidation half-cycles.
-
Inhibition and deactivation study.
-
Enzyme immobilization.
-
Research on the optimal reactor system and system for the extraction of reaction products.
-
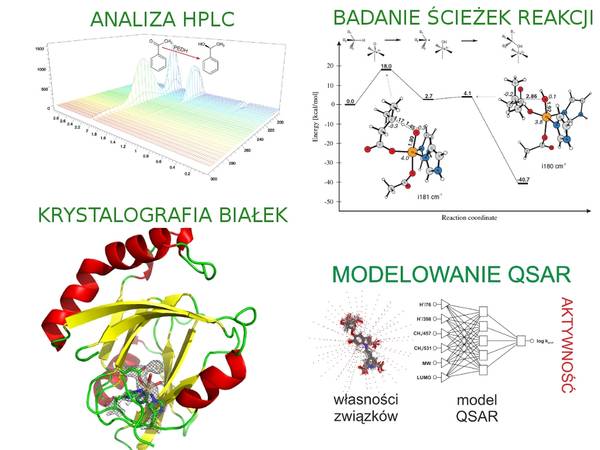
Modeling reaction pathways with quantum chemical methods (QM and QM:MM).
-
Modeling enzyme-substrate complexes with molecular docking and molecular dynamics simulations.
-
Modeling enzymatic reactivity with Quantitative Structure Activity Relationship (QSAR) approach.
- X-ray protein crystallography.
- Research on bacterial production of polyhydroxyalkanates (PHAs)
- Studies of functionalization of PHA polymers with selected drugs from the group of antibiotics, steroids and non-steroidal anti-inflammatory drugs by biocatalysis
- Esterification of sugars with PHA monomers – (R)-3-hydroxy acids
METHODS
- Spectroscopic techniques for studying enzyme activity with various substrates based on a photometric activity test (UV/Vis). Studies of the half-cycle reaction cycle using the methods of fast stopped-flow kinetics in cooperation with the Bioinorganic Chemistry Team prof. Grażyna Stochel from the Faculty of Chemistry of the Jagiellonian University.
- FPLC and electrophoresis techniques for enzyme purification.
- LC-MS and GC-MS with RP and chiral columns for separation of reaction’s products and identification of enzyme stereospecificity.
- Liquid-liquid and solid phase extraction (SPE) of reaction mixtures for products purification.
- Isothermal titration calorimetry for investigation of thermodynamics of enzyme reaction.
- Quantum chemical modeling of enzyme active sites and reaction mechanisms
- Molecular dynamics and docking simulations for enzyme-substrate complexes.
- Prediction of biological activity with artificial neural networks and QSAR equations based on quantum chemical parameters.
- X-ray crystallography.
- Structural roentgenography.
- Microbial fermentation in flasks and fermenter (5L)
SELECTED PROJECTS
EBDH
Ethylbenzene dehydrogenase (EBDH) is a bacterial enzyme belonging to mononuclear molybdenum enzymes from the DMSO family of reductases. EBDH is the first key enzyme in the anaerobic ethylbenzene mineralization pathway that enables bacteria to grow on a nutrient medium rich in ethylbenzene and nitrates in an anaerobic environment.
Molybdenum enzymes feature a unique active site containing molybdenum atom, one or two pteridine cofactors and additional ligands (i.e. amino acid residues of Ser, Cys, SeCys or Asp and very often an oxo ligand).
|
molybdenum cofactor |
EBDH structure |
EBDH is a complex bacterial enzyme (αβγ 164 kDa) containing a monomolybdenum center (MoCo), iron-sulfur clusters and a b559 heme group. EBDH catalyzes the stereospecific hydroxylation reaction of ethylbenzene to 1-(S)-phenylethanol.
HppE
(S)-2-hydroxypropylphosphonic acid epoxysidase (HppE) is a bacterial enzyme that catalyzes the last stage of biosynthesis of phosphomycin, an antibiotic used in clinical practice. HppE belongs to non-hem iron enzymes, which means that in its active place it binds an iron ion without the participation of a porphyrin group. For the native substrate – S-HPP, the catalyzed reaction consists in the oxidative dehydrogenation of the substrate with the simultaneous formation of an epoxy ring. The R-HPP stereoisomer is more easily oxidized to ketone. The research is aimed at eluciding the mechanism of the enzymatic reaction of HppE.
|
Reactions catalyzed by HppE |
Structure of the Active Site HppE |
Polyhydroxyalkananes
Polyhydroxyacalcates (PHAs) are biopolymers that represent one of the leading sectors of bioproducts. PHAs represent a class of optically active biodegradable polyesters accumulated by numerous bacteria as isolated intracellular granules or as extracellular lattice-like structures. PHAs serve primarily as a reserve of coal and energy. The chemical structure of PHA can be described as a linear formation of (R)-3-hydroxy acids, where a carboxyl group of one monomer forms an ester bond with a hydroxyl group of an adjacent monomer. PHA can be divided into several categories depending on the criterion adopted. Taking into account the size of the monomer, PHA is divided into short (C3–C5; scl-PHA), medium (C6–C14; mcl-PHA) or long > (C14; lcl-PHA) polymers in chains.
Chemical structure of PHA, where R1 and R2 are different side chains
Our research focuses on PHA polymers as well as their monomers. The ongoing work involves biocatalysis to functionalize PHA polymers and its monomers. We use enzymes from the group of α/β-hydrolases – lipase, thanks to which we create new materials based on PHA. Bioplastic is subjected to enzymatic functionalization with selected drugs, on the other hand, monomers by biocatalysis are attached to sugar molecules. The research aims to create new materials for tissue regenration, dressing materials, new drugs with anti-cancer effects or surfactants.
Four research projects are carried out on a given subject:
- THE NCN Sonata project “Biosynthesis of new lactose esters via lipases. Characterization of their physicochemical and anti-cancer properties.” No. 2015/17/D/ST4/0051
- Know Project “Biocatalytic synthesis of sugar esters”
- NCBiR Leader Project“New functionalized biopolymers for medical applications”No. 0090/L-7/2015
- TECHMATSTRATEG project “Technology of biorefining vegetable oils for the production of advanced composite materials”No. 407507/1 / NCBR / 2019