Development and industrial implementation of the biotechnological method of vitamin D3 hydroxylation and its derivatives
Calcifediol – a versatile drug
Currently, calcifediol is obtained by chemical methods (i.e., a multi-stage organic synthesis). The Polish synthesis of calcifediol was developed in the 1980s by the Pharmaceutical Institute in Warsaw [2]. This multi-stage process requires a lot of work and time, has a low final yield (approx. 20%), and requires a complicated procedure for the purification of the final compound.
Unexpected discovery
The ICSC PAS has developed a method for the synthesis of calcifediol from vitamin D3 (cholecalciferol) by means of a one-step regioselective hydroxylation. The process uses enzyme preparation as a catalyst and chemical re-oxidants. The synthesis process itself is simple to carry out and easy to translate on a large scale. However, the production of a biocatalyst requires more advanced microbiological (large-scale bacterial cultivation) and biotechnological methods (biocatalyst separation). However, thanks to the use of a separate biocatalyst, a very high-purity product is obtained in the synthesis.
The enzyme that enables the synthesis of calcifediol is steroid C25 dehydrogenase (S25DH), an enzyme derived from the bacteria Sterolibacterium denitrificans. This bacterium can consume cholesterol even under anaerobic conditions. It means that bacteria cannot use molecular oxygen to oxidize this compound and therefore has to use quite unusual biocatalysts. One such atypical enzyme is S25DH. S25DH catalyzes the hydroxylation reaction i.e., introduces the OH group exclusively at the twenty-fifth carbon atom within the side chain of cholesterol and its derivatives. This means that the catalyzed reaction is regioselective, that is, it takes place only at one point in the chemical molecule. Surprisingly scientists have found that the enzyme can hydroxylate not only cholesterol derivatives but also vitamin D3. And it all started with completely basic research, an attempt to answer the question of how steroid C25 dehydrogenase works.

Homological model of the catalytic subunit of the S25DH enzyme and the interior of its active center containing the molybdenum cofactor.
Basic research – the beginning of the journey
The adventure of scientists from ICSC PAS with steroid C25 dehydrogenase began with theoretical research, where in the Theoretical and Experimental Biocatalysis group, Professor Maciej Szaleniec conducted research on molybdenum enzymes. S25DH is precisely such an enzyme and that is why prof. Szaleniec was invited to join a research project conducted by microbiologists from the University of Freiburg in Germany. Theoretical studies enabled an understanding of how the “enzyme heart” – the active center in which the hydroxylation reaction takes place – is built and how the enzyme oxidizes the molecule despite the lack of access to molecular oxygen [3]. The enzyme turned out to be so interesting that prof. Szaleniec, that he also decided to start experimental research and learn about the activity of S25DH with various substrates. Together with a group of Ph.D. students and young scientists he started biochemical characterization of S25DH. The potential for industrial application of the enzyme was especially studied by dr Agnieszka Wojtkiewicz who selected this topic for her doctoral dissertation. Although the first reports in the literature indicated that the enzyme was practically inactive with vitamin D3, after optimization of the reaction conditions dr Wojtkiewicz proved that such hydroxylation can be successfully performed. Moreover, it turned out that the enzyme hydroxylates vitamin D3 faster than its natural substrate [4].
Applied research and industrial implementation
Applied research was financed by the National Center for Research and Development as part of the LIDER project. Thanks to the funds from the National Center for Research and Development, scientists from ICSC PAS developed a method of enzyme purification (easy to implement in the industry), optimized the form of the catalyst (so that it was durable and cheap), and checked what other valuable chemicals can be hydroxylated by the enzyme. After the completion of the NCBiR project, prof. Szaleniec and dr Wojtkiewicz continued their work on the use of S25DH in the synthesis of calcifediol. A very important step was to increase the scale of synthesis i.e., the transition from small test tubes used in the laboratory (with a volume of 0.5 to 2 ml) to reactors used in the industrial synthesis of valuable drugs. These studies were carried out in cooperation with the Pharmaceutical Institute in Warsaw, which allowed for trial syntheses on a 5-liter scale. In the following years, the Institute acquired an industrial partner that financed the implementation project. This allowed testing of the method on an even larger scale and verification of whether the biotechnological method of synthesis is competitive with the chemical method used. This project optimized the conditions of culture bacteria that produce the enzyme. Bacterial growth takes place in the so-called fermenters on a scale similar to the industrial one (40-120 liters). A method of preparing large amounts of catalyst (5-10 liters) was also developed. Ultimately, the implementation research was successful and ICSC PAS sold the license to an industrial partner.
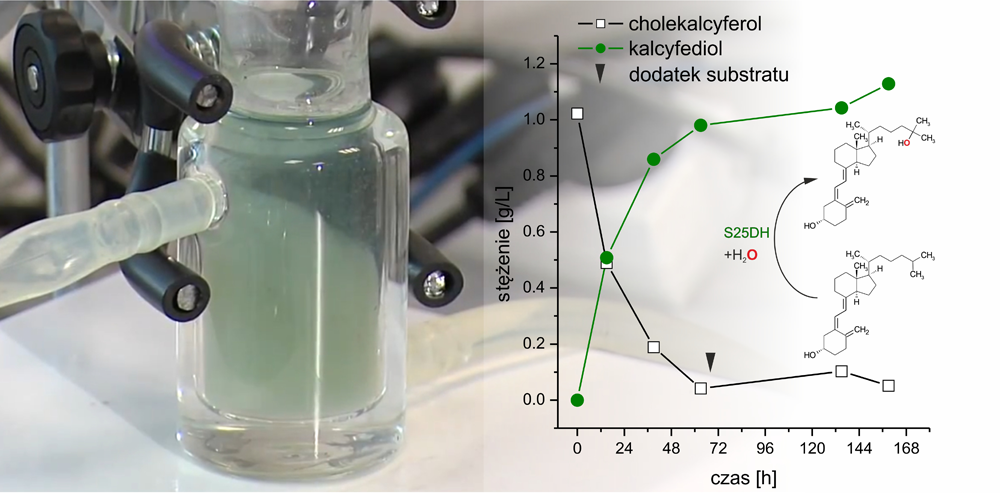
Hydroksylacja witaminy D3 do kalcyfediolu w reaktorze laboratoryjnym
And what’s next?
Of course, the question arises, what is the continuation of this story? Unfortunately, some aspects of it must still be kept secret and the Institute is not yet able to share its details with you. At least until the calcifediol produced with a method developed by the Institute appears on pharmacy shelves. So please keep your fingers crossed for us. Our scientists are constantly working and supporting industrial partners the biotechnological production of a generic drug could be launched soon.
References
[1] M. Entrenas Castillo, L.M. Entrenas Costa, J.M. Vaquero Barrios, J.F. Alcalá Díaz, J. López Miranda, R. Bouillon, J.M. Quesada Gomez, “Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study”, J. Steroid Biochem. Mol. Biol. 203 (2020) 105751.
[2] T. Ryznar, M. Krupa, A. Kutner, “Syntheses of vitamin D metabolites and analogs. Retrospect and prospects”, Przem. Chem. 81(5) (2002) 300-310.
[3] A. Rugor, A. Wójcik-Augustyn, E. Niedzialkowska, S. Mordalski, J. Staroń, A. Bojarski, M. Szaleniec, “Reaction mechanism of sterol hydroxylation by steroid C25 dehydrogenase – Homology model, reactivity and isoenzymatic diversity”, J. Inorg. Biochem. 173 (2017) 28–43
[4] A. Rugor, M. Szaleniec, J. Staroń, “Sposób otrzymywania 25-hydroksylowanej witaminy D3 (kalcyfediolu)”, Polish Patent PL 235932 (2020)