An unexpected discovery – a new tungsten hydrogenase
Maciej Szaleniec and his team at the Jerzy Haber Institute of Catalysis and Surface Chemistry, Polish Academy of Sciences
A few years ago, I submitted a grant proposal to the European Research Council, seeking funding from this most prestigious research institution in Europe. I wanted to study an enzyme derived from bacteria that live near volcanic vents, which contains a tungsten atom at its core. To my despair, the grant application was crushed by a critical review. The expert concluded that no discovery had been made in the proposed field in the past 25 years, and therefore saw no reason to fund my research. A few years later, it became clear just how wrong that expert was…
Hydrogen – Not Just the Fuel of the Future
We all place high hopes in hydrogen as an alternative fuel. Climate change and environmental pollution caused by burning fossil fuels will sooner or later force a transformation in our civilisation. Meanwhile, hydrogen is a clean fuel— when burned, it is transformed only into water. What’s more, it’s abundantly available around us. Although trapped in water molecules, it covers 70% of our planet’s surface, and we already know how to release it from water via electrolysis, which can be powered by solar energy. Furthermore, it seems that there are vast underground deposits of hydrogen that can be mined and used.
Interestingly, hydrogen is not only a valuable fuel. It’s also an important substance for the chemistry of the future, especially if we stop processing crude oil. For over 150 years, the majority of our chemical industry has relied on oil to produce chemicals, plastics, solvents, cleaning agents, and medicines. In the future, this must change, as oil reserves are not limitless.
Even today, chemists and biotechnologists are working on using chemicals found primarily in plants so that we can replace oil—for instance, with cellulose or lignin from wood pulp or other plant biomass, particularly waste material. An added benefit of this shift is that trees create wood by absorbing carbon dioxide from the atmosphere, helping to combat climate warming.
There are, however, some challenges with chemicals derived from wood. They contain too many oxygen atoms to be used, for example, as fuels. Take cellulose, the main component of wood. It’s a sugar—also known as a carbohydrate—which means each carbon atom is paired with one oxygen and two hydrogens, just like in water (H₂O). Our chemical industry, however, is accustomed to processing hydrocarbons—compounds made mostly of carbon and hydrogen atoms.
So, is there a way to connect these new renewable chemical sources from nature with our current industrial methods? The answer is yes! It’s possible to “reduce” the oxygen content in these substances—that is, remove oxygen atoms and convert them chemically into compounds more similar to petroleum derivatives.
One such eco-friendly way to perform this reduction is to treat the substances with hydrogen, which in the future we’ll also obtain via sustainable methods. However, to conduct hydrogen-based reduction efficiently, we need catalysts—materials that accelerate chemical reactions and allow us to transform substances exactly as we need.
Although research into catalytic hydrogenation has been ongoing for years and we already have some industrial catalysts, there is still much to discover, especially in the field of biocatalysis.
Tungsten Aldehyde Oxidoreductase from Anaerobic Bacteria
And here we return to my titular “unexpected discovery.” Despite not securing the prestigious ERC grant, we managed to obtain a small grant from the Polish National Science Centre for my PhD student, Agnieszka Winiarska. Together with my colleagues from Germany, we began studying a tungsten aldehyde oxidoreductase that originated from the bacterium Aromatoleum aromaticum. As the name of the bacterium suggests, this organism is capable of feeding on aromatic hydrocarbons
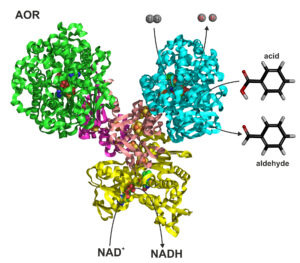
The title for Picture 1: Tungsten-containing aldehyde oxidoreductase. Tungsten atoms are located in the catalytic subunits marked in green and blue.
Tungsten oxidoreductase (Picture 1) is used by bacteria to oxidise aldehydes into carboxylic acids. Aldehydes are toxic substances—they’re the reason we suffer headaches after a night of heavy drinking. When our enzymes metabolise ethanol into acetaldehyde, it poisons the body and triggers the unpleasant symptoms of a hangover. Similarly, bacteria that “consume” and process various chemicals sometimes produce toxic aldehydes that they need to eliminate quickly.
As it turned out during our research, the enzyme we studied serves precisely this purpose—it detoxifies bacterial cells by oxidising aldehydes into much safer acids. Interestingly, it performs this task without using molecular oxygen, accelerating reactions in a completely different way than enzymes in oxygen-respiring organisms.
Studying anaerobic enzymes requires anaerobic chambers—large boxes equipped with rubber gloves that allow scientists to manipulate objects in an oxygen-free environment (Picture 2). Both in my laboratory and in my German colleagues’ lab, we use the same type of chambers filled mainly with neutral nitrogen and a hint of hydrogen. Because the chambers aren’t completely airtight, we place a special catalyst inside that uses hydrogen to reduce any remaining oxygen, converting it into water.

Picture 2: Working with anaerobic enzymes requires a special glove box filled with an oxygen-free atmosphere. In our device, this atmosphere consists of nitrogen with a small admixture of hydrogen.
During her stay in Marburg, my doctoral student studied the enzyme inside such a chamber. Her main task was to observe how quickly it oxidises various aldehydes into organic acids. To monitor the reaction, we use spectrophotometers—devices that can detect changes in the colour of liquids. After returning to Kraków, she continued her research in our chamber. To her surprise, the solution containing the enzyme began changing colour even before she added any aldehyde that should have been oxidised. The reaction seemed to occur spontaneously, as if by some mysterious alchemical process.
She had never witnessed anything like this during her research stay in Germany! Naturally, it turned out not to be magic—besides the inert nitrogen, another gas was floating inside the chamber: hydrogen, which nobody had suspected. Gases dissolve in liquids, so hydrogen came into contact with the enzyme, which then oxidised the hydrogen and interacted with a dye in the solution, causing the colour change.
And so, in this rather accidental way, we discovered a new hydrogenase!
Hydrogenases – Enzymes That Are Close Friends with Hydrogen
Hydrogenases are metalloenzymes capable of both oxidising hydrogen—that is, separating the protons from the electrons—as well as synthesising hydrogen molecules from protons present in solution and electrons. Until now, known hydrogenases contained either one or two iron atoms, or a combination of nickel and iron atoms in their catalytic core. So far, no enzyme catalysing such a reaction using a tungsten atom has been discovered.
Hydrogenase activity is highly attractive to the biotechnology industry. It allows for the release of electrons contained in a hydrogen molecule and the reduction of other chemical compounds. In our case, the use of hydrogen enables the reversal of a natural reaction—that is, carboxylic acids can be converted into aldehydes.
But why would anyone want to produce toxic aldehydes? First, not all aldehydes are toxic. Many of them have very pleasant aromas and are highly valuable additives in food and perfumes. A good example is the compound responsible for the delightful smell of vanilla—vanillin aldehyde. Our enzyme enables its production from vanillic acid.